Tag: quality
Why choose Quest Diagnostics
Quest Diagnostics is the only SAMHSA-certified and CAP-FDT-accredited laboratory to offer in-house testing for all three of the most popular drug test types: urine, oral fluid, and hair.
Drug test collections at-a-glance
Quality collections play a key role in the drug testing process. We provide insights into the important role of trained collectors and our nationwide collection network.
Clarifying the changes in federal workplace drug testing & documentation
Changes to the federal drug testing panel and paperwork are forthcoming, but for now you should continue testing as usual.
Revisions to Federal workplace drug testing
HHS revised its mandatory guidelines to expand federal urine drug testing to include hydrocodone, hydromorphone, oxycodone, and oxymorphone.
We’re There When You Need IT: Web services
Quest Diagnostics web services provide off-the-shelf and custom integrations into your HRIS or ATS.
We’re There When You Need IT: ESP
The Employer Solutions Portal is a prime example of the way Quest Diagnostics incorporates customer needs and feedback into developing IT solutions.
Time tested technology
Technology must be reliable to consistently deliver time and cost savings. Our system availability, or up-time, averages between 99.61% and 99.98%.
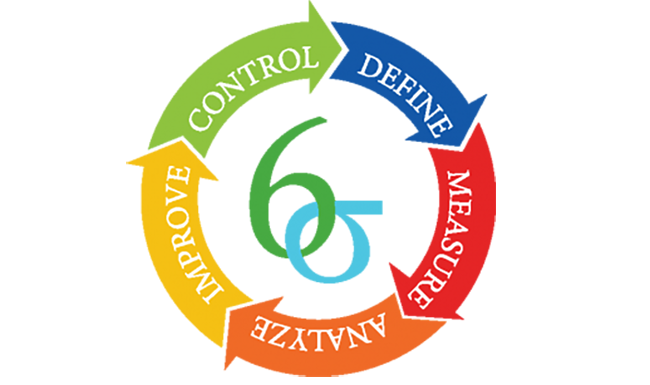
Ask the Experts: Six Sigma quality
At Quest Diagnostics, Six Sigma means striving for a standard of quality near perfection and championing total customer satisfaction.
Express Results Online collection quality
Express Results Online renders reliable and accurate results within minutes at a nearby Quest Diagnostics drug test location.
Drug test collections – Our focus on quality
At Quest Diagnostics, we hold our collection sites and team to the highest standards of quality and professionalism.