Tag: HHS
Proposed guidelines for hair drug testing
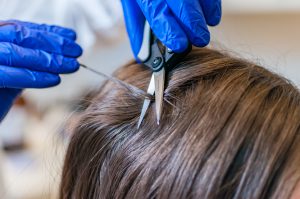
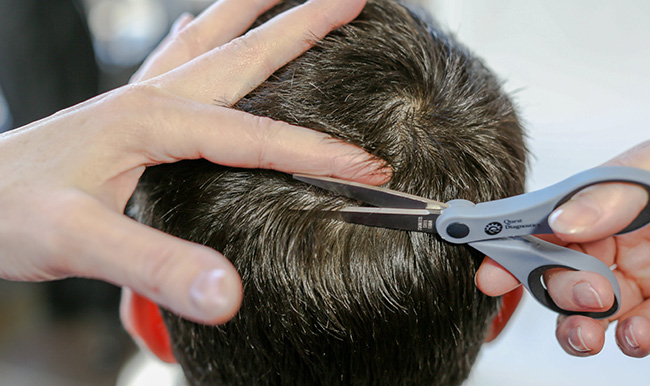
SAMHSA has proposed scientific and technical guidelines for the inclusion of hair in Federal drug testing programs. Note: These are only proposed Guidelines and hair testing is not approved for any Federally mandated drug (eg, DOT) testing. Read all public comments online including those from Quest.
New Federal CCF coming soon
SAMSHA/OMB have requested public comments to revisions of the current Federal CCF for workforce drug testing. However, Quest anticipates a one-year implementation period for continued use of the form and will help prepare clients for the switch.
Clarifying the changes in federal workplace drug testing & documentation
Changes to the federal drug testing panel and paperwork are forthcoming, but for now you should continue testing as usual.
Revisions to Federal workplace drug testing
HHS revised its mandatory guidelines to expand federal urine drug testing to include hydrocodone, hydromorphone, oxycodone, and oxymorphone.
Important changes with eCCF
eCCF impacts the entire federally-regulated drug testing process. Read about important changes and how they can affect your drug testing program.
Speed up testing with eCCF
Employers can begin taking advantage of eCCF for their DOT urine drug testing.
Quest Diagnostics Approved to Use eCCF for Federally-Mandated Workforce
Benefits of eCCF include fewer data entry and legibility issues, reduced collection site flaws, less paper to manage and improved overall efficiency.
eCCF update: The paperless revolution
Federal eCCF inspections are complete and all Quest Diagnostics drug testing labs are approved to process electronic Custody and Control Forms.
Regulated electronic Custody and Control Form (eCCF) progress update
We are excited to begin offering eCCF for regulated programs, but like any product, we perform rigorous quality assurance processes before we do.
Quest comments for hair specimen mandatory guidelines
HHS published an RFI seeking comments for federally-mandated hair drug testing. Here are our comments in response to the questions posed by HHS.