Oral-Eze achieves significant milestone
<
>
Related
Email Newsletter
Stay up-to-date with the latest news and information from the drug testing industry by subscribing to Results, our monthly newsletter.
 Your Privacy Choices
|
Privacy Notices
|
Terms
|
Language Assistance / Non-Discrimination Notice | Asistencia de Idiomas / Aviso de no Discriminación | 語言協助 / 不䈚視通知
Your Privacy Choices
|
Privacy Notices
|
Terms
|
Language Assistance / Non-Discrimination Notice | Asistencia de Idiomas / Aviso de no Discriminación | 語言協助 / 不䈚視通知
Quest, Quest Diagnostics, any associated logos, and all associated Quest Diagnostics registered or unregistered trademarks are the property of Quest Diagnostics. All third-party marks — ® and ™ — are the property of their respective owners. © 2000-2026 Quest Diagnostics Incorporated. All rights reserved.

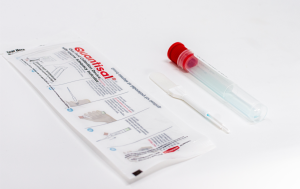















On September 1, 2011, Quest Diagnostics introduced Oral-Eze® to the drugs of abuse testing marketplace. To date, hundreds our drug testing clients have successfully transitioned to Oral-Eze, making it their sole oral fluid drug testing device.
As clients have transitioned, we’ve seen Oral-Eze volumes in our laboratory continue to increase. At the same time, volumes of the device we’re moving away from have steadily declined over time. April 19, 2012 marked the first day that we tested more Oral-Eze specimens than we did the old device. Since that day, we’ve continued to see steady increases in Oral-Eze volume. Today, Oral-Eze represented nearly 60% of the more than 3,000 oral fluid drug tests we screened.
“With its blue sample adequacy indicator, and no unpleasant taste, Oral-Eze is satisfying collectors and donors alike,” said Dr. Barry Sample, Director of Science and Technology. “When asked if she thought the conversion to Oral-Eze was going to be a challenge for her collectors, a program manager from one of our transportation clients simply responded, ‘Certainly not. The Oral-Eze collection process is a piece of cake.’ We couldn’t have said it better ourselves.”
For more information, visit our website. or contact us online.