Quest Diagnostics to exclusively test Oral-Eze
<
>
Related
Email Newsletter
Stay up-to-date with the latest news and information from the drug testing industry by subscribing to Results, our monthly newsletter.
 Your Privacy Choices
|
Privacy Notices
|
Terms
|
Language Assistance / Non-Discrimination Notice | Asistencia de Idiomas / Aviso de no Discriminación | 語言協助 / 不䈚視通知
Your Privacy Choices
|
Privacy Notices
|
Terms
|
Language Assistance / Non-Discrimination Notice | Asistencia de Idiomas / Aviso de no Discriminación | 語言協助 / 不䈚視通知
Quest, Quest Diagnostics, any associated logos, and all associated Quest Diagnostics registered or unregistered trademarks are the property of Quest Diagnostics. All third-party marks — ® and ™ — are the property of their respective owners. © 2000-2026 Quest Diagnostics Incorporated. All rights reserved.

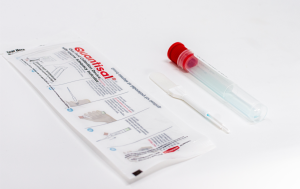



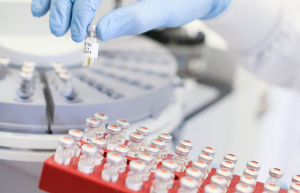












We encourage you to make the transition today, and to start enjoying the benefits of Oral-Eze.
For more information, visit Oral-Eze.com or contact us online.