Tag: we’re there
I’m There: Danielle Coyle
Danielle helps the laboratory in Norristown, PA prioritize specimens and processes. She is invested in making a difference in the world.
I’m There: Gina Roberts
As a scientist at Quest Diagnostics, Gina Roberts, comes to work each day motivated to help solve some of the complex challenges facing our business.
I’m there: Jewel Williams
In our Atlanta laboratory, Jewel Williams personifies enthusiasm and uses that energy to help her team care for each drug test specimen.
I’m There: Joe Yoest
Joe Yoest, Toxicology Confirmations Supervisor, has been a familiar face in the laboratory since its construction in 1999. He reflects on the drive of our technicians that leads to tremendous growth.
I’m there: Anthony Magazzolo
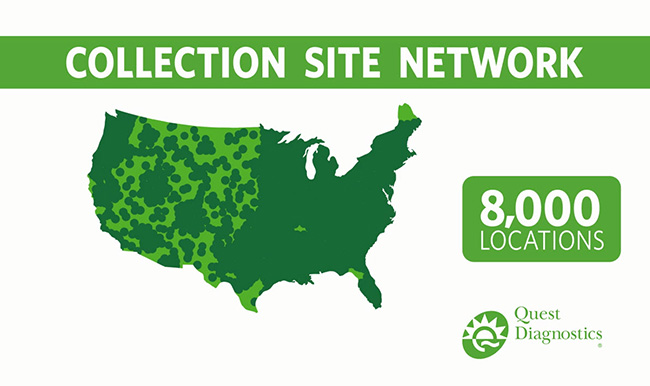
Anthony Magazzolo nurtures strong connections throughout our business to help our extensive, nationwide, drug testing collection network run smoothly.
I’m there: Kristina Dawn
Kristina believes in the power of people’s energy. She uses it to help motivate, while building an inclusive and positive environment.
I’m there: Liz Vokolek
Liz Vokolek believes that by personally owning the problem and the solution, we can create a more productive work environment and a better customer experience.
I’m there: Lori Reinhardt
Lori Reinhardt believes that by working together we’re better positioned to overcome obstacles and resolve challenges. In this month’s feature of our “I’m There” series, Lori gives us her take on what ‘we’re there when you need’ us means to her. Read the full story.
Drug test collections video
Our most recent video highlights our different drug test collection services and the benefits of each.
Electronic Chain of Custody Forms and regulated testing
eCCF advantages include fewer data entry and legibility errors, reduced paper consumption, and specimen tracking throughout the testing process.