Category: Drug Testing
Employers are asking about oral fluid drug testing options, but why now?
Even though oral fluid drug testing has been around for more than 2 decades, many employers are taking it seriously for the first time.
Quest Diagnostics hair testing now includes specific testing for Fentanyl and Methadone
Recent enhancements to our hair testing panels now allow for more specific testing for Fentanyl and Methadone.
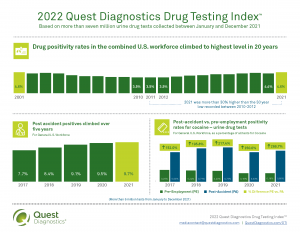
How to use the Quest Diagnostics Drug Testing Index™ to make important drug-free workplace decisions
The DTI has practical application when considering the best options for a drug-free workplace drug testing program.
What Every Employer Should Know About State Drug Testing Laws: Part 2
Not every state has a drug testing law, but most have workers’ and unemployment compensation regulations with drug testing laws language.
What Every Employer Should Know About State Drug Testing Laws: Part 1
Employers have many questions about drug testing, That’s in part because no 2 state drug testing laws are exactly alike.
Old Federal CCFs… Now what?
All U.S. Department of Transportation (DOT)-mandated drug test collections will require a new Custody and Control Form. Find out what you need to do to be prepared for the fast-approaching change.
Avoid testing delays – transition to the new DOT CCF
Current DOT CCFs are expiring soon. Proactively prepare for this change and double-check your paper CCF inventory today. Act now to avoid testing delays.
Why test for marijuana?
Here are 4 critical questions every employer should be asking when it comes to maintaining a safe and healthy drug-free workplace.
Oral Fluid Drug Testing and Emerging Technologies
Oral fluid testing remains an important part of a drug-free workplace program with many benefits from the form of collection to industry-wide appeal.
Navigating drug testing in the great resignation
It's more important than ever for employers to offer competitive hiring processes to find the best possible employees for their workplace.